Heparin Sulfate Arrays to Probe Binding Protein Specificity
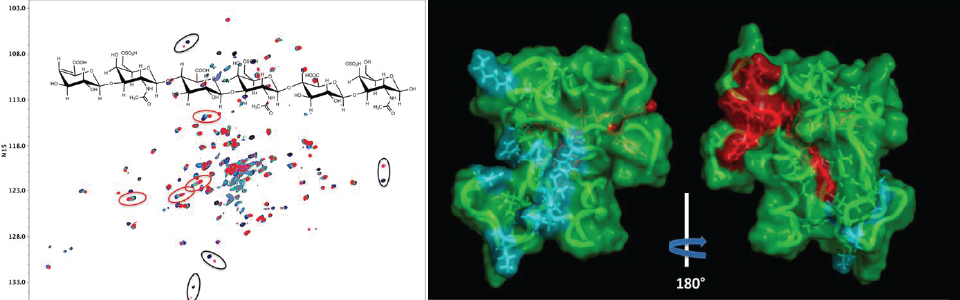
Although hundreds of heparan sulfate binding proteins have been identified, and implicated in a myriad of physiological and pathological processes, very little information is known about ligand requirements for binding and mediating biological activities by these proteins. This difficulty results from a lack of technology for establishing structure-activity-relationships, which in turn is due to the structural complexity of natural HS and difficulties of preparing well-defined HS-oligosaccharides. To address this deficiency, we have developed a modular approach for the parallel combinatorial synthesis of HS oligosaccharides that utilizes a relatively small number of selectively protected disaccharide building blocks that can easily be converted into glycosyl donors and acceptors. These compounds can then be employed for the assembly of a wide range of HS-oligosaccharides.
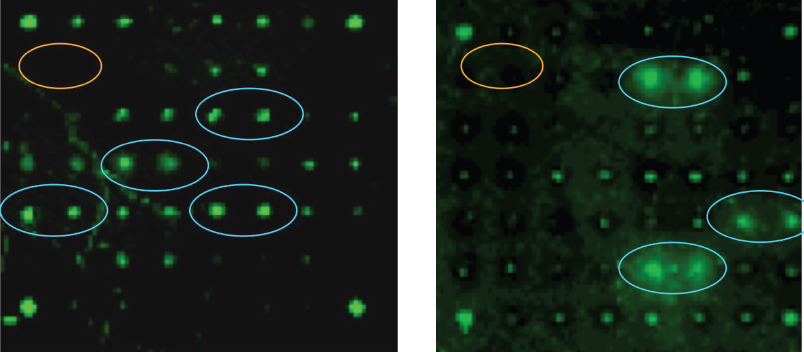
The synthetic HS oligosaccharides are equipped with aminopropyl spacer, which made it possible to attach the compounds to microarray plates having reactive carboxylic acid moieties. The resulting HS oligosaccharide array is being further developed for high throughput screening to establish ligands for HS-binding proteins. In collaboration with several research groups, the synthetic HS-oligosaccharides are also employed as standards to develop mass spectrometry (MS)-based sequencing methods.