TSG-6 Link Module Displays Multiple modes of Glycosaminoglycan Binding
Link modules found in a number of cell surface proteins play important roles in stabilizing the extracellular matrix (ECM) by interacting with glycosaminoglycans (GAGs). However the specificity and geometry of these interactions is poorly defined. Driven by a collaborative project with the Tony Day lab of the University of Manchester, Younghee Park in the Prestegard group has applied forefront NMR methods to characterize binding modes of the link module of TSG-6, the secreted protein product of tumor necrosis factor-stimulated gene-6. A combination of chemical shift perturbation and paramagnetic relaxation enhancement, using defined and chemically modified oligomers of chondroitin sulfate, has identified two different binding sites (one strong and one weak). The second site appears to be formed after GAG induced dimerization of the Link module. The multiplicity of binding sites clearly can promote crosslinking and stabilization of the ECM.
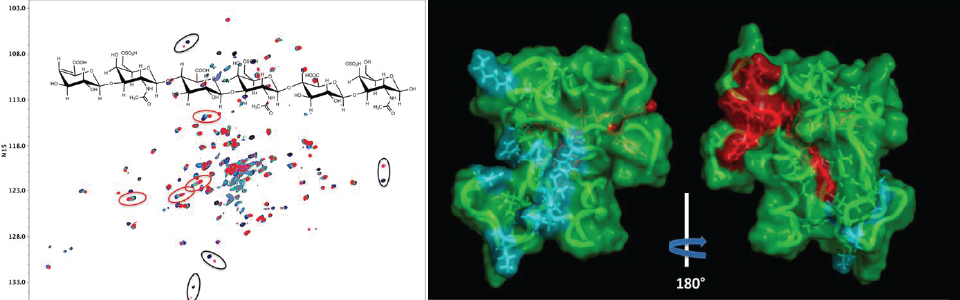
Red and black ellipses on overlaid 1H-15N HSQC spectra, collected with increasing amounts of the hexameric chondroitin sulfate oligomer, show chemical shift patterns characteristic of fast and slow exchange. Fast and slow exchange residues are highlighted in red and blue respectively on the structure.
